Page 7 - hypertension_newsletter9
P. 7
REFLECTIONS
Hypertension
Hypertension Global Newsletter #9 2025
Lorundrostat, a selective aldosterone synthase inhibitor, has entered late-stage development as a potential new therapeutic option.
The phase 3 Launch-HTN randomised clinical trial evaluated the efficacy and safety of lorundrostat in adults with uncontrolled Hypertension
hypertension on two to five background medications, including those with TRH. This study aimed to provide evidence supporting the
use of lorundrostat as a new treatment option for this challenging patient population, as well as address the limitations of existing
aldosterone-targeted therapies.
A total of 1083 adults with a mean age of 61.6 years, consisting of 46.9% female participants, employed a 12-week, double-blind,
placebo-controlled design. Participants were taking stable doses of two to five prescribed antihypertensive medications at baseline,
with 60.1% taking three or more. The intervention randomised participants (in a 1:2:1 ratio) to receive daily placebo (n = 272),
50 mg/d of lorundrostat for 12 weeks (n = 541), or 50 mg/d of lorundrostat for six weeks followed by a possible escalation to 100
mg/d for the final six weeks (n = 270), provided they met specific safety and efficacy criteria. The primary outcome was change in
automated office systolic blood pressure (AOSBP) at six weeks.
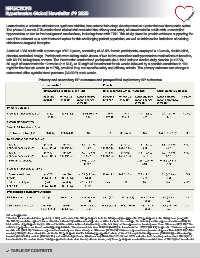
Primary and secondary BP outcomes and prespecified exploratory BP outcomes
NA, not applicable.
a The data for lorundrostat were pooled (n = 808) and included the 538 participants from the 50 mg-only group plus the 270 participants in the 50 mg dose group with
the 100 mg escalation option. Unless otherwise indicated by a different sample size or with a footnote. For example, the data may not be expressed as mean (SD); the
b
total number of participants may be different from the number in the column heading, or the data may not be expressed as least-squares mean difference (95% CI). The
c
estimated proportions are based on multiple imputed data. The observed data were 332/755 (44.0%) for lorundrostat vs. 62/257 (24.1%) for placebo. Data are expressed
d
as odds ratio (95% CI). Data are expressed as least-squares mean change or difference (97.5% CI). Data are expressed as regression slope coefficient (95% CI). After
f
g
e
receiving 50 mg/d of lorundrostat for six weeks, 92 participants met the following prespecified criteria at Week 6 for systolic blood pressure (130 mmHg) and levels of po-
tassium (4.8 mmol/L), sodium (135 mmol/L), and estimated glomerular filtration rate (>45 mL/min/1.73 m2 and <25% reduction from baseline) and received 100 mg/d of
lorundrostat for the next six weeks. Met prespecified criteria at six weeks; after six weeks, only these participants received the escalated dose of 100 mg/d of lorundrostat.
h
TABLE OF CONTENTS